A new study published by scientists at Columbia University Medical Center and AZCERT in the Journal of the American College of Cardiology and reported on in a recent Chicago Tribune feature story describes a new way of conducting research, learning from what most now call “big data” by linking the analyses of different types of data from multiple sources including basic research. The approach demonstrates tremendous potential by identifying dangerous drug interactions that have so far been undetected. As a leader in drug interaction research, AZCERT’s founder Dr. Raymond Woosley helped design the experiments and some of the data analytic methods for this research. Information provided by the non-profit AZCERT’s website, CredibleMeds.org, was essential for researchers to find a “needle in a haystack” in regards to detecting potentially harmful drug interactions. The diagram, shown here from the editorial that accompanied the report, represents how data from multiple sources can now be analyzed and used to pinpoint previously hidden drug interactions.
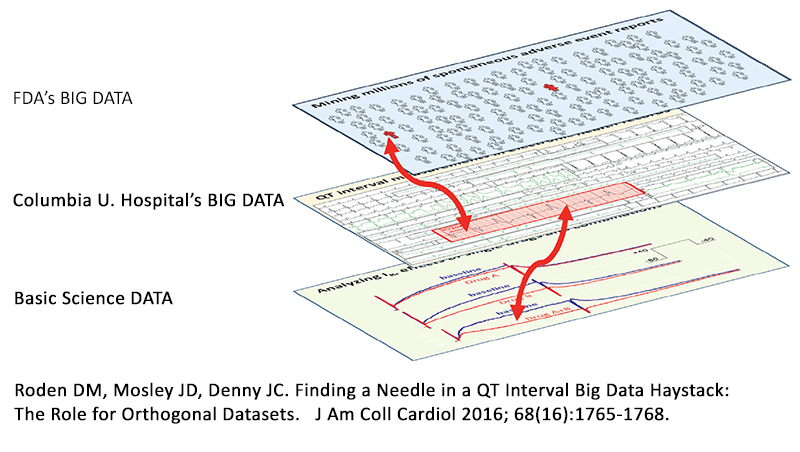
This new information has been made possible by a new algorithm developed by scientist Dr. Nicholas Tatonetti, that analyzes data from Columbia University Hospital, FDA and basic science experiments. This is an important breakthrough in patient safety and AZCERT is proud that the drug safety information provided on the CredibleMeds.org website contributed to this effort. The website is maintained under a contract with the US Food and Drug Administration’s Safe Use Initiative and supported Dr. Woosley’s participation in this research.
See additional Comments at: http://www.practiceupdate.com/content/coupling-data-mining-and-laboratory-experiments-to-discover-drug-interactions-causing-qt-prolongation/45065/65/2/1